How To Calculate Oh Concentration
G formula chemistry Solved 3. calculate the oh concentration in a 1.0 x 10-3 m Calculating ph from [oh-] hydroxide concentration
Solved: 5) Calculate The Concentration Of OH' In A Solutio... | Chegg.com
Given [h+] or [oh-], calculate ph & poh Calculating ph using hydroxide ion concentration, [oh-] Concentration calculate example
Solved the value of kw at 50 c is 5.48 x 10-14. calculate
Solved calculate the oh concentration and ph of a 0.0020 mCalculating hydroxide ion concentration Calculate concentration oh chegg transcribed text showSolved calculate the oh- concentration in an aqueous.
Calculate weight, mass, concentration, volume and ph of aqueous solutionsSolved calculate the concentration of oh" and the ph value Calculate oh concentration solvedCalculate transcribed.
![Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE](https://i.ytimg.com/vi/gn1CgBzShps/maxresdefault.jpg)
Concentration ion hydroxide calculating
Concentration gcseCalculate concentration ph solution weight mass molarity poh value calculation Calculate concentration oh value solution kw aqueous h3o solved problemOh h3o calculate.
Ph concentration oh hydroxide calculating simpleOh concentration calculate ba solution Calculate [oh-] or [h3o+]Calculating poh using oh- concentration.

Solved: 5) calculate the concentration of oh' in a solutio...
Concentration hydroxide ion ph oh calculating usingPh concentration calculate nagwa hydroxide hydrogen aqueous ions calculating Concentration oh aqueous calculate solution h3o has degree problem solved transcribed text show please doHow to calculate ph from oh concentration : may 29, 2020 · how to.
Ph poh calculations oh calculate given worksheetPh calculate concentration hydroxide ions ion worked methods usin two Concentration calculate h3o homeworklib 0x10Calculate the concentration of h3o⁺ in a solution that contains 5.5 ×.

Poh oh calculating
Calculate concentration (example)How to calculate ph from oh concentration : may 29, 2020 · how to .
.


Calculating pOH using OH- Concentration - YouTube

Calculate Weight, Mass, Concentration, Volume and pH of Aqueous Solutions

Solved Calculate the OH concentration and pH of a 0.0020 M | Chegg.com

Solved Calculate the OH- concentration in an aqueous | Chegg.com
![Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)
Given [H+] or [OH-], Calculate pH & pOH - YouTube

Solved: 5) Calculate The Concentration Of OH' In A Solutio... | Chegg.com

G Formula Chemistry - pametno
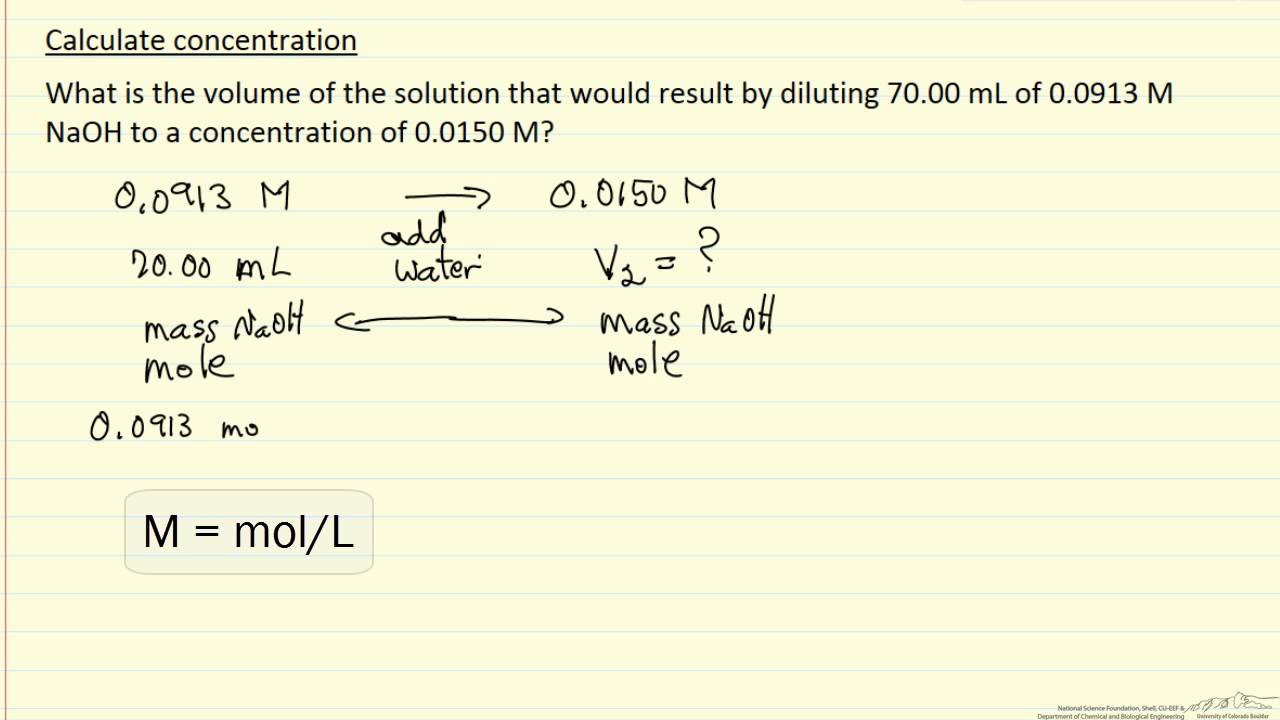
Calculate Concentration (Example) - YouTube